Organic Chemistry II Laboratory for Majors
A.K.A. How to make carbon-carbon bonds with carbanions, cations, carbenes, radicals, and pericyclic reactions!
Spring 2026
Text: Experimental Organic Chemistry A Small-Scale Approach, 2nd Edition, Wilcox & Wilcox, ISBN 0-02-427691-X
Please note that the scale of each experiment may be changed.
James Salvador, jsal at utep dot edu, ext. 5704, contact me via email to setup a Microsoft Teams meeting.
Tuesday Teaching Assistants:
CCSB 1.0506, Jared Goldman, jsgoldman at miners dot utep
dot edu
CCSB 1.0508, Vanessa Aitken, vbaitken at miners dot utep dot edu
Thursday Teaching Assistant:
CCSB 1.0506, Paulo Dos Santos, prdossantos at miners dot utep dot edu
|
|
 |
| Week | Dates | First Activity | Second Activity |
| 1 | January 20 & 22 | No TAs, no labs | |
| 2 | 27 & 29 | Laboratory Safety-Moodle | |
| 3 | February 3 & 5 | Benzaldehyde page 75 | Steam Distillation page 293, reference Chapter 7 |
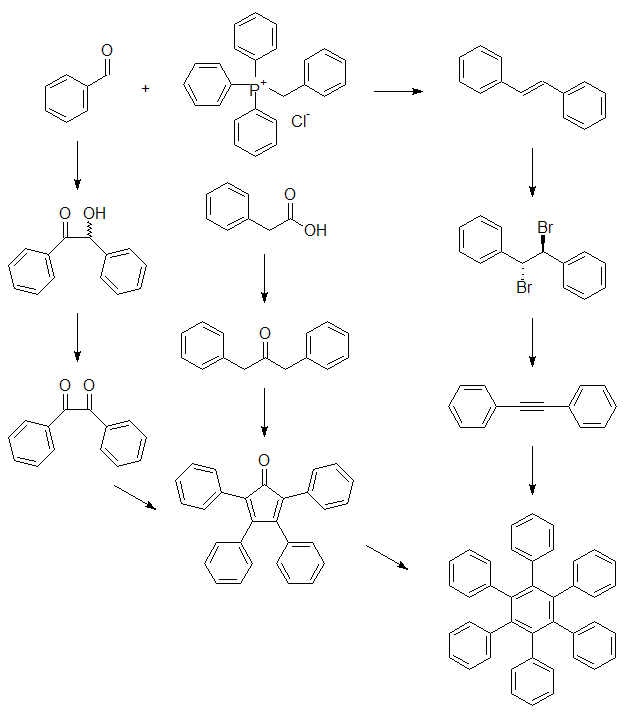
| 4 | 10 &12 | Stilbene page 369 | Carbene-Chapter 47 |
| 5 | 17 & 19 | Stilbene continued | Binaphthols-Chapter 46 |
| 6 | 24 & 26 | Stilbene Dibromide page 370 | Pinacol-Chapter 37 |
| 7 | March 3 & 5 | Diphenylacetylene page 370 | Pinacol continued |
| 8 | 10 &12 | Benzoin page 481 | Beckmann-Chapter 25 |
| 9 | 17 & 19 | Holiday | Holiday |
| 10 | 24 & 26 | Benzoin continued | Friedel Craft Acylation-Chapter 32 (B) |
| 11 | March 31 & 2 | Benzil page 482 | Wolff-Kischner-Article |
| 12 | 7 & 9 | Dibenzyl Ketone-Article | Electrophonic Aromatic Substitution-Article |
| 13 | 14 & 16 | Tetraphenylcyclopentadienone page 400 | Carboxylation-Chapter 36 |
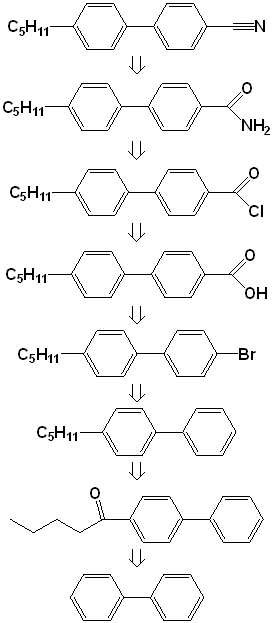
| 14 | 21 & 23 | Hexaphenylbenzene page 438 | Amide, page 193-4 |
| 15 | 28 & April 30 | 5-CB-Article | |
| 16 | 5 & 7 | LCD Manufacture | |
Required materials (You will be turned away and earn a zero in attendance for missing safety attire!):
- The book
- Goggles (not safety glasses) A seal should form around your eyes.
- Pants (not shorts) Your legs must be covered.
- Shoes (not sandals) Your feet must be completely covered.
- A lab coat
What you should be learning:
- Know the dangers of each laboratory including how to mitigate your risk.
- Read and understand all the chapter and not just the experimental procedure.
- Make sure that you can draw all structures and mechanisms for each lab.
- Be able to apply a given mechanism to other reagents because we are not just cooks!
- Understand which fundamental mechanism you are applying (Addition, Elimination or Substitution) including the stereochemical consequences.
- Understand whether a reaction is an oxidation, reduction or not a net redox reaction.
- Be able to calculate the yield of a
reaction.
- structure to formula conversion,
- formula to molecular weight conversion,
- ml to grams via density and vice-versa,
- grams to moles,
- what is the limiting reagents,
- what is the stoichiometry of the reaction.
- Understand why a particular procedure was followed.
Your grade will consist of:
- 1/3 attendance. You must participate fully in the lab, and not come and go as you please!
- 1/3 laboratory Pre-Lab Quizzes. Pre-Lab Quizzes will be open only on the Monday of the given lab week.
- 1/3 laboratory Post-Lab Quizzes. Post-Lab Quizzes will open on the Friday morning after the lab and close at 11:59 pm of the next Monday.
A > 89.5 %, B > 79.5 %, C > 69.5 %, D > 59.5 %

 T
T